Innovating with Compliance: Jacoti Hearing Center's Trailblazing Approach to EU Health App Regulations
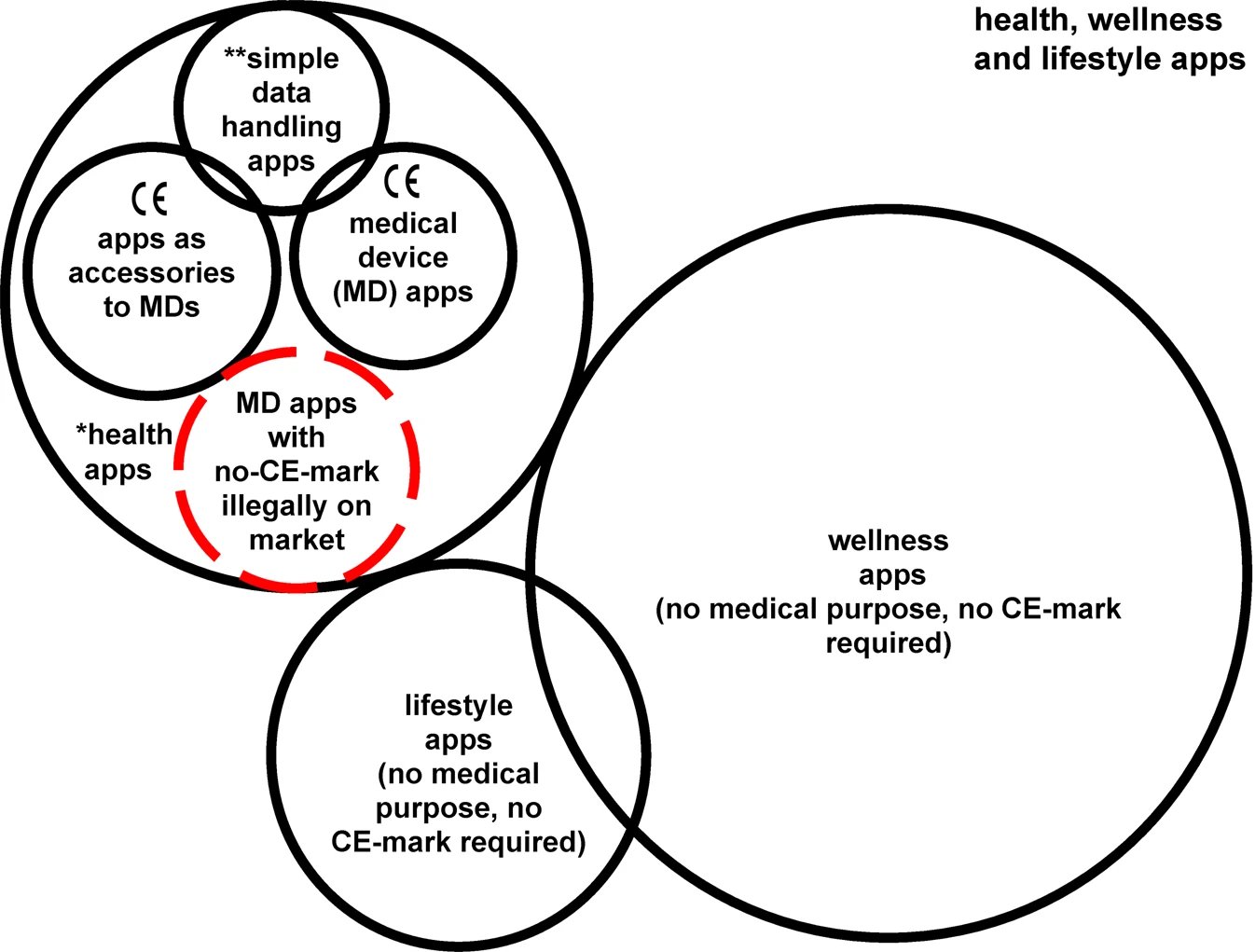
"Fig. 1: Categories of apps in the health, wellness, and lifestyle area" from Sadare, O., Melvin, T., Harvey, H. et al. Can Apple and Google continue as health app gatekeepers as well as distributors and developers?. npj Digit. Med. 6, 8 (2023). doi.org/10.1038/s41746-023-00754-6. Copyright © 2023, The Author(s) About Jacoti Jacoti BV | Hearing Technologies is a science-based company that develops hearing enhancement solutions embeddable in consumer devices. Its flagship product, Jacoti Inside, optimizes audio to each individual hearing requirement from consumer technologies to fully-fledged medical devices. For more information visit jacoti.com
Contact our Press Officer for more information or to arrange an interview with our team.
press@jacoti.comIn the rapidly evolving landscape of digital health and wellness apps, Jacoti Hearing Center stands out as a shining example of compliance with the stringent regulations imposed by the European Union (EU). As Apple and Google navigate the complexities of ensuring app compliance with medical device regulations, Jacoti Hearing Center has already established itself as a pioneer in adhering to these requirements.
As distributors of thousands of health and wellness apps in the EU, Apple and Google are now required to ensure compliance with medical devices regulations and report any significant incidents resulting from app usage, following recent changes in EU law. However, the level of adherence by application developers to these new rules varies and additionally it raises uncertainties, regarding conflicts of interest as Apple and Google simultaneously function as importers, distributors, and developers of their own competitive health products. Moreover, the proposed European Health Data Space Regulation suggests a shift towards voluntary registration and labelling of wellness apps, treating them more like medical devices than consumer software.
The article Can Apple and Google continue as health app gatekeepers as well as distributors and developers? published in Nature explores the implications of these regulations and proposes future models that emphasize the need for enhanced approaches to facilitate the evolution of safer, superior, and fairer digital health applications throughout the EU. Furthermore, the implementation of EU legislation could serve as a blueprint for global application in other regions.
Jacoti's commitment to providing safe and reliable health software is evident in its proactive approach to compliance. Jacoti has diligently ensured that Jacoti Hearing Center meets the necessary medical device regulations and quality management standards, including the Conformité Européenne (CE) mark through MDR Release as Class IIa device, besides being also listed as a Class II FDA Medical Device.
Looking ahead, Jacoti continues to set the benchmark for compliance in the digital health industry and the responsible consumerization of solutions that impact consumers' hearing sense. This proactive approach, without taking shortcuts, coupled with their commitment to user safety, positions Jacoti as a trusted and reliable provider of global hearing enhancement software within the EU market and beyond.
References:
Sadare, O., Melvin, T., Harvey, H. et al. Can Apple and Google continue as health app gatekeepers as well as distributors and developers?. npj Digit. Med. 6, 8 (2023). https://doi.org/10.1038/s41746-023-00754-6
Jacoti Achieves MDR Certification – The Strongest Base for High-Quality Consumer Audio Tech